Neurophysiology of Pain: Physiological and Sensory Responses The neural mechanisms by which pain is perceived are part of a complex neurophysiological process. Most patients facing the end-of-life transition have pain. Nurses must understand the physiological process and the person�s sensory perception of the process in order to assure that the patient has appropriate therapies to obtain pain relief. Selection of effective pharmacological and nonpharmacological therapies requires consideration of the holistic nature of pain, which is a multidimensional experience. Multidimensional FrameworkThe multidimensional view of pain is most explanatory of clinical pain for which nurses assume responsibility to relieve.Current conceptual understanding views pain as a multidimensional phenomenon with four dimensions.
Types of pain (Acute, Chronic, Malignant versus
Nociceptive, Neuropathic)
Pathophysiologic Consequences of Unrelieved Pain Unrelieved pain is more than an annoyance; it is physically and psychologically dangerous.� Many of the dangerous effects of unrelieved pain are the same as the effects of opioid analgesics.� Knowing that pain not relieved can have dangerous consequences should help nurses to better advocate for appropriate analgesic therapies.
Such knowledge emphasizes the need for a new model of pain control. The model focuses on cure of pain when possible, preemptive analgesia for all pain and palliation of pain when its cure is not possible. Treatment of the underlying cause of the pain is the primary focus of medical and nursing care, but when its cure is not possible, the treatment focus shifts to palliative pain relief as the primary focus. A preemptive approach to pain anticipates when pain will be experienced and appropriate therapy instituted in an attempt to prevent or at least minimize the experienced pain. Evidence in support of preemptive analgesia is inconclusive.� Studies have been designed with insufficient power or other methodogical flaws that limit conclusive proof of the preemptive analgesia concept. The notion of palliation of pain is important when the pathophysiologic consequences of unrelieved pain are considered. Palliative pain control is a concept that began with the hospice movement and control of cancer pain. Today, the concept can apply to most pain including postoperative pain, burn pain, trauma-related pain, chronic nonmalignant pain, and pain related to terminal illness. Palliative treatment of all pain minimizes the pathophysiologic consequences of unrelieved pain and reinforces high quality of care to all people. Palliative pain control is a concept that apply to people facing the end of life, but it also applies to all people who experience acute or chronic types of pain. Application of this new model that includes palliative care of all pain requires understanding of the neural mechanisms of nociceptive and neuropathic types of pain. Pain Mechanisms: The "Pain Process"
and Blocking it with Analgesics and Nonpharmacological Strategies The nurse uses knowledge of the pain mechanisms to interpret assessment data and to select therapies that promote maximum pain relief with minimum side effects. The neural mechanisms by which pain is perceived involve a process that involves four major steps:
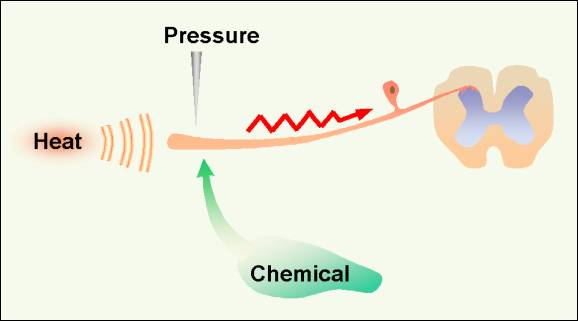
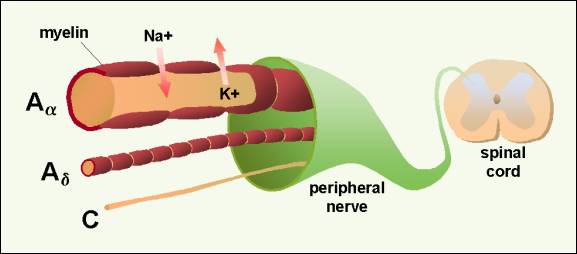
The transduction and transmission steps relate to the neurochemical signals of actual or impending tissue damage (nociceptive stimuli). Not all nociceptive stimuli are perceived as pain. If there is sufficient modulation of signals and perception of nociceptive events is prevented, there is no pain. Perception is critical to sensing pain. Modulation, either enhancing or inhibiting nociception, therefore is crucial to pain perception. Most pain management techniques probably mimic endogenous pain inhibition processes. Conversely, pain that is difficult to relieve probably results from enhanced nociceptive signals. Additional details about these four steps provide a foundation for nursing practice. Transduction Figure 1.�� Conversion of chemical, heat or presure stimuli into a neuronal action potential is the first step in the pain process, transduction.� 2001 D.J. Wilkie, used with permission. Anatomy and Physiology of Transduction The A-alpha and A-beta fibers carry the sensation of light pressure to deep muscles, soft touch to skin, and vibration. The A-alpha and A-beta fibers primarily ascend to rostral centers in the dorsal column pathway, but they also make synapses in the spinal dorsal horn close to synapses of the A-delta and C fibers (Figure 2). This dorsal horn connection means that input from touch fibers can enter the spinal cord and synapse or communicate with cells carrying nociceptive input.� Figure 2.� Sensory nerve with large myelinated A-alpha fiber carrying touch sensation, A-delta and C fibers carrying pain sensation to dorsal horn of the spinal cord.� 2001 D.J. Wilkie, used with permission. The three types of fibers differ in size and speed at which action potentials are conducted.1 A-alpha or A-beta fibers are large (6 to 22 microns) with myelin sheaths around them. Because of the myelin sheath and axon size, A-alpha and A-beta fibers conduct at a rapid rate (35 to 120 meters per sec). In contrast, A-delta fibers are smaller fibers also with myelin sheaths. Because of their size (1 to 5 microns), A�delta fibers conduct at a slower rate (5 to 30 meters per sec) than the larger A-alpha and A�beta fibers. C fibers, in comparison, are small (0.2 to 1.5 microns) and unmyelinated. C fibers occur singly or in clusters, and they conduct at a rate of 0.5 to 2 meters per sec. The conduction rates are important because information carried to the spinal cord by the A-alpha and A-beta fibers will communicate with dorsal horn cells sooner than information carried by A-delta or C fibers. These conduction rates have important implications for modulation of noxious information from A-delta and C fibers. Because of anatomical proximity, the peripheral environment (physiologic milieu) of the axons and dendrites of one neuron can influence other nearby neurons. Pathophysiology of Transduction Burns are obvious examples of tissue damage from thermal stimuli. Superficial burns injure somatic tissue and result in nociceptive pain. Massive burns, however, are likely to injure peripheral nerve fibers as well as somatic tissues. Therefore, extensive burns may result in both neuropathic and nociceptive types of pain. Tissue damage produces chemicals that cause an action potential.2� Although it is intuitively clear how pressure from masses or injury from burns initiate the neuronal action potential, it may be less obvious how chemicals generate an action potential. Therefore, chemical transduction is described in detail because of its importance to pain experienced by acutely ill patients. Figure 3. Diagram summarizing ingredients of peripheral soup in response to tissue injury. A. Direct activation of primary afferrent nociceptor (PAN) by intense pressure and consequent cell damage which leads to potassium (K+) leading from the cell and to prostaglandin (PG) and bradykinin (BK) synthesis. B. Secondary activation occurs when impulses generated in the stimulated terminal produce a retrograde release of peptides, such as substance P (SP). SP causes vasodialation increased accumulation of bradykinin, and realse of histamine (H) from mast cells and serotonin (5HT) from platlets. C. Histamine and serotonin levels rise in the extracellular space, sensitizing nearby nociceptors. Sympathetic efferent fibers (SEF) release norepinephrine (NE) and additional prostaglandins, which activates the nociceptor if the nerve has been injured.Adapted from: Howard L. Fields, MD, PhD, Pain �(New York: McGraw-Hill Book Company, 1987). Permission: McGraw Hill Book Company. When tissue is traumatized and cells are damaged, a number of chemicals are released near the PAN (Figure 3). Some chemicals (bradykinin, serotonin, histamine, potassium ions, norepinephrine) stimulate while others (leukotrienes, prostaglandins, substance P) sensitize the PAN to be excitable and to fire an action potential toward the spinal cord. Several details are helpful in fully understanding this process and how analgesics affect it. All human cells have a two-layer lipid membrane. When a cell is damaged, phosolipids and other substances are liberated from the cell into the intracellular space. The release of phosolipids initiates the arachidonic acid cascade through which 5-lipo-oxygenase and cyclo-oxygenase synthesize leukotrienes and prostaglandins, respectively.3 These events are displayed in Figure 3.� Leukotrienes and prostaglandins sensitize the PAN to be activated by a smaller stimulus than when these chemicals are not near the PAN. For example, light pressure is not perceived as painful in normal conditions, but sometimes is sensed as pain (allodynia) if leukotrienes or prostaglandins surround the PAN. Figure 4.� Inhibition of the arachidonic acid pathway by several drugs. Steroids inhibit production of arachidonic acid, thereby blocking synthesis of leukotrienes and prostaglandins. Ketoprophen is believed to block production of 5-lipo-oxygenase and cyclo-oxygenase. Aspirin and nonsteroidal anti-inflammatory drugs block conversion of cyclo-oxygenase to prostaglandins and thromboxane A2. Trilisate blocks production of prostagandins E2 and F2, but not I2 or thromboxane A2.� 2001 D.J. Wilkie, used with permission. Inhibition of leukotriene and prostaglandin synthesis can improve pain control when tissue damage is known or suspected (Figure 4).� A specific inhibitor of leukotrienes is not yet approved for pain control, but ketoprophen, a nonsteroidal anti-inflammatory drug (NSAID), appears to have some activity in blocking leukotriene synthesis.4� Aspirin and other nonsteroidal anti-inflammatory drugs block prostaglandin synthesis, and most interfere with platelet aggregation. Choline magnesium trislicylate (TrilisateTM), however, is an example of a NSAID that does not inhibit synthesis of thromboxane A2, a necessary factor for platelet aggregation.5� Steroids act earlier in the cascade and prevent production of arachidonic acid, thus inhibiting synthesis of both leukotrienes and prostaglandins. Any of these drugs (ASA, NSAIDs [including COX1 and COX2] and steroids) block chemicals that sensitize the PAN and thereby raise the threshold at which the PAN is transduced. These drugs are powerful analgesics, particularly when there is tissue injury such as occurs with excessive peripheral edema, burns, arthritis, and bone metastasis. In addition to the arachidonic cascade, many chemicals activate the PAN when they leak out of the cell or are released into the intracellular space as part of the inflammatory response.6� For example, potassium and histamine exude from damaged cells, and bradykinin is degraded from plasma kininogen, a component of inflammatory exudate. Other chemicals are released from platelets (serotonin) or mast cells (histamine). Sufficient concentrations of any one of these chemicals around the PAN will cause the PAN to be activated (transduced), firing an action potential. These chemicals also act in combination to sensitize the PAN, enabling it to fire with a stimulus smaller than usual. Few drugs inhibit the excitatory actions of these chemicals.� Antihistamine drugs are an exception. By blocking the excitatory action of histamine, these drugs have shown analgesic effects in people with cancer.7 If the PAN is activated and fires an action potential, the PAN itself releases chemicals, one of which is substance P.6 Substance P is stored in the distal terminals of the PAN and is released through a retrograde process.� In this way, substance P sensitizes the PAN, dilates nearby blood vessels, which leads to local edema and causes release of histamine from mast cells. Capsaicin (ZostrixTM), when applied to the skin in the painful area, is believed to deplete the PAN terminal of substance P and, by this action, to block retrograde release of substance P.6 In this way capsaicin, a topical analgesic available over the counter (OTC) helps to prevent further sensitization of the PAN. Finally, activation of the autonomic nervous system contributes to PAN transduction through release of norepinephrine and prostaglandins.8 Norepinephrine activates a PAN when it comes into contact with the PAN, only if the PAN has been injured.9 Tumors often invade nerve tissue and surgical incisions cut small peripheral nerves, both of which can produce injured PANs (examples of neuropathic pain). In the context of nerve injury, therefore, it is clear that emotions, which can increase autonomic nervous system release of norepinephrine, can increase pain though physiologic mechanisms. Nonpharmacologic (behavioral) and pharmacologic methods of reducing autonomic nervous system activation can be important analgesic methods. For example, reducing fear, anger, and anxiety through behavioral methods (such as patient education, relaxation, and distraction), theoretically can reduce activation of the PAN. Anxiolytic agents also can reduce activation of the PAN by reducing autonomic nervous system discharge. In summary, tissue injury results in production and release of a number of chemicals around the PAN. These chemicals can sensitize or activate the PAN directly (example of nociceptive pain) and through secondary processes (usually an example of nociceptive pain unless neural tissue has been injured, then an example of neuropathic pain). These chemicals are commonly described as ingredients in the peripheral soup surrounding the PAN. If any or all of these ingredients can be eliminated from the peripheral soup, then the PAN may not send an action potential to the CNS. A number of non-opioid analgesics inhibit these chemicals and thereby remove peripheral soup ingredients that contribute to PAN activation. Drugs that block production or release of these chemicals can be powerful analgesics and are the first line drugs recommended in several published guidelines for acute pain and cancer pain.10-13 In addition to the powerful effect of the drugs inhibiting peripheral soup ingredients, people with pain often engage in behaviors that limit PAN activation. For example, if a certain movement routinely produces pain, a person will avoid that movement. Guarding and immobilizing the injured body area are common methods of preventing the onset of pain.14, 15 One way patients guard against pain is to wear loose, unrestrictive clothing when light touch produces pain (allodynia). Use of a foot cradle is another way to remove the pressure of a sheet or blanket when the weight causes pain. Using canes, walkers, or back braces are examples of ways to prevent PAN activation in a person with vertebral metastasis. These types of devices prevent movement-induced vertebral flexion, a common source of pain in people with vertebral masses (bone metastasis) or vertebral lesions (osteoporosis). Such behavioral methods of pain control can supplement drugs that also prevent transduction. Some pain control behaviors, however, may have untoward sequelae. For example, the person with a chest or abdominal incision who guards the respiratory muscles by taking shallow breaths will dramatically increase his/her risk of atelectasis and pneumonia.16 Long-term immobilization can increase the person�s risk of developing a decubitus ulcer. Persons with pain are quite expert at finding behavioral methods to prevent or minimize PAN activation. Recognition of these behavioral pain control attempts helps the nurse to avoid the common misconception that a patient lying quiet and still can not be experiencing pain. When a patient minimizes activity, the nurse should administer regularly scheduled not PRN analgesics in order to prevent the undesired sequela of the immobilization. Transmission Projection to the CNS Figure 5. Propagation of a neuronal action potential
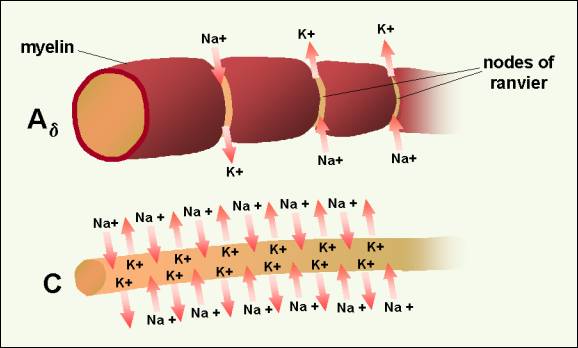
in a C fiber (bottom) and a myelinated fiber (Ad) (top).�
Because of myelinization, ion exchange occurs only at the nodes of Ranvier. The action potential can be inhibited, however, if the ion channels are inactivated. Drugs known as membrane stabilizers inactivate the sodium channels and disrupt the transmission of the action potential along the PAN axon.18 Some adjuvant drugs, such as local anesthetics (e.g., lidocaine, bupivicane, mexilitine, EmlaTM) and anticonvulsant drugs (e.g., phenytoin, carbamazepine, clonazepam), prevent transmission via this type of mechanism. In dilute concentrations, local anesthetics effectively block small fiber transmission. Larger concentrations of local anesthetics block larger fibers, including the motor fibers.19 An important concept to recognize is that one nerve cell extends the entire distance from the periphery to the dorsal horn of the spinal cord. The cell usually makes synapses only at the terminals at the peripheral and central nervous system sites. For example, an afferent fiber from the great toe travels from the toe through the 5th lumbar nerve root into the spinal cord; it is one cell. It does not synapse at the knee or hip. Once an action potential is generated, it travels all the way to the spinal cord. The message will be transmitted to the dorsal horn of the spinal cord, unless it is blocked (e.g., by a sodium channel inhibitor) or disrupted (e.g., by a lesion at the central terminal of the fiber such as a dorsal root entry zone lesion {DREZ}). Altering the peripheral soup ingredients at the distal end of the PAN is an important way to prevent pain. Once the first nerve cell in the pain process has fired an action potential, however, the uninhibited message will be transmitted to the spinal cord. 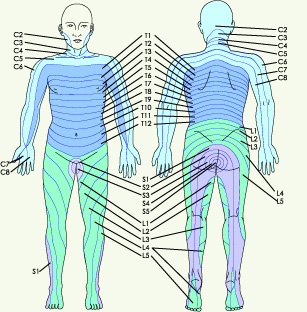 Figure 6.� Spinal dermatomes represent organized sensory input
carried via specific spinal nerve roots. S=sacral, L=lumbar, T=thoracic,
C-cervical. � 2001 D.J. Wilkie, used with permission. The A-alpha, A-beta, A-delta, and C fibers extend from the peripheral tissues through the dorsal root ganglia to the dorsal horn of the spinal cord (Figure 2). The area of skin innervated by a single nerve root is known as a dermatome (Figure 6). Each nerve root innervates typical segments of the body, sometimes far removed from the area where the nerve enters the spinal cord. Although fibers enter the spinal segment associated with the nerve root in which they travel to the spinal cord, the A-delta and C fibers send dendrites rostrally (toward the brain) and caudally (toward the feet) for two to four spinal segments6 (Figure 7). Therefore, one fiber can communicate with as many as 9 spinal segments. The innervation expanse is important when transcutaneous nerve stimulators (TENS) are used to block transmission of nociceptive stimuli.� If, in positioning TENS electrodes, the nurse does not take into consideration the full range of spinal segments innervated by a single nerve cell, pain may not be blocked. Figure 7.� Each A-delta and C fiber sends dendrites rostrally (toward the brain) and caudally (toward the feet) for two to four spinal segments. One fiber can communicate with neurons in as many as 9 spinal segments.� 2001 D.J. Wilkie, used with permission. Dorsal Horn Processing
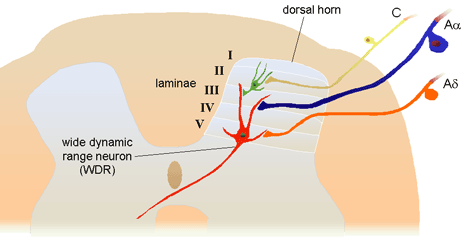
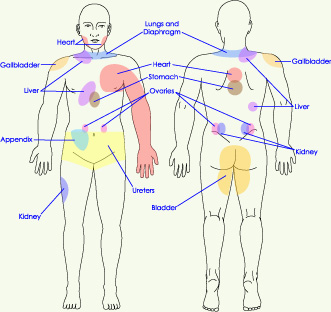
Figure 8a.� Sensory input to dorsal horn projection and interneuron cells. Lamina I projection neurons are predominately excited by nociceptive input directly from A-delta fibers and indirectly from C fibers.� 2001 D.J. Wilkie, used with permission.� Figure 8b.� Sensory input to dorsal horn projection cells in Lamina V, wide dynamic range (WDR) that receive input from low-threshold A-beta fibers as well as direct and indirect input from nociceptive afferents. Dendrites of the WDR neurons also receive input from nociceptive (Lamina II) and non-noxious (Lamina III) cells.� 2001 D.J. Wilkie, used with permission. Neurotransmitter release and binding involves several different types of cells located in the dorsal horn (Figure 8a and 8b).� Areas within the gray matter of the spinal cord are represented by laminae numbered between I and X; the dorsal horn is represented by laminae I to V.� Cells that receive nociceptive input are located in Lamina I (projection cells), Lamina II (some projection cells and interneurons), and Lamina V (wide dynamic range neurons most of which are projection cells).6 All of the cells in these laminae are important to propagation of the nociceptive signal from the spinal cord to the brain. Most projection cells (second-order neurons) send axons to the brain in the contralateral anterolateral quadrant. They receive excitatory and inhibitory messages, the net sum of which determines whether the PAN action potential will be transmitted to the brain on the opposite side of the stimulus. Interneurons can be either excitatory or inhibitory. They communicate with other lamina II cells, located within one or two spinal segments, and with dendrites from cells located in laminae I, III, IV, and V.2 The concept of excitatory and inhibitory interneurons is important, because it helps to explain why some behavioral therapies are effective. Although the exact mechanisms have not been determined, it is known that stimulation of large sensory fibers (A-beta) can have an inhibitory effect on cells that project nociceptive signals to the brain. TENS and massage are examples of nonpharmacologic methods by which rapidly conducting large fibers can be activated. Application of heat or cold are examples of methods by which smaller, less rapidly conducting fibers can be activated by non-noxious stimuli. All of these methods are known to inhibit transmission of nociceptive stimuli.� Wide dynamic range (WDR) neurons receive input from noxious stimuli primarily carried by A-delta and C fiber afferents (especially from viscera), non-noxious stimuli from A-beta fibers, and indirect input from dendritic projections into laminae I, II, III, and IV.6 Most lamina V neurons project to the brainstem and to the thalamus.1, 2 Discovery that WDR neurons have large receptive fields and receive inputs from non-noxious and noxious stimuli provides a neural explanation for referred pain.6, 20 Inputs from nociceptive fibers innervating visceral organs and non-noxious somatic fibers innervating the body part to which pain is referred all converge on the same WDR neuron. When the message received by the WDR neuron is transmitted to the brain, the originating location is poorly localized. Pain, therefore, is perceived in the body part presumably innervated by the somatic fiber rather than from the visceral A-delta or C fibers. Since primary and metastatic malignant tumors often involve thoracic, abdominal, and pelvic viscera, the concept of referred pain must be considered when interpreting the location of pain. As indicated in Figure 9, the location of the pathology may be quite distant from the pain location reported by the patient. For example Figure 9 shows that pain from liver disease is located in the right upper abdominal quadrant, but also it frequently is referred to the anterior and posterior neck region and to a posterior flank area.� If referred pain is not considered when evaluating a pain location report, therapy could be misdirected.�
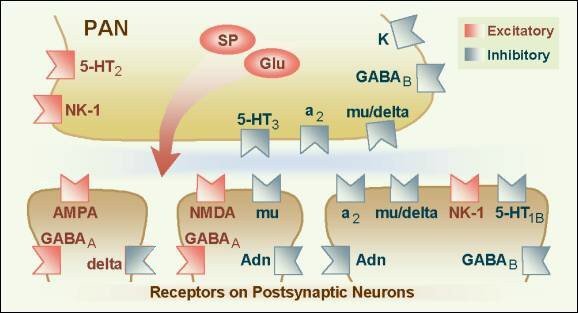
Figure 9. Posterior and anterior views showing commonly reported sites wehre pain is referred from visceral organs.� 2001 D.J. Wilkie, used with permission. An important concept related to neural mechanisms of persistent pain is neural plasticity.21 Repetitive transmission of PAN nociceptive signals to the dorsal horn results in several changes in dorsal horn processing, including: 1) enlargement of the receptive field of a peripheral neuron, 2) activation of receptors normally inactive, 3) 'wind-up' of C fibers (a phenomenon of progressively increased neural response to repeated noxious stimuli), and 4) allodynia (pain evoked by light tactile stimulation).2, 21, 22 N-methyl-D-aspartate (NMDA) and non-NMDA receptors are excitatory and have been implicated in these changes in dorsal horn processing.21 The NMDA receptors are enabled once the neuron has been depolarized and along with subsequent changes in calcium channel conduction, produce profound alterations in neural processing of afferent stimuli (an example of neuropathic pain) that can persist for long periods of time (Figure 10). For this reason, current pain treatment goals are to be preemptive and prevent nociceptive pain and, therefore, the neural changes (causing neuropathic pain) that occur with a persistent barrage of nociceptive stimuli.1 Although research is being conducted to develop NMDA antagonist drugs for clinical use, two currently available drugs, dextromethorphan (cough suppressant) and ketamine (general anesthetic), block the NMDA receptor-mediated changes.1 Methadone is now believed to have NMDA antagonist activity. Figure 10.Wilcox�s hypothetical spatial arrangement of excitatory and inhibitory receptors and transmitters on the primary afferent nociceptor (PAN) and postsynaptic neurons in the spinal dorsal horn. � 2001 D.J. Wilkie, used with permission. Propagation through the CNS Generally, the STT and SRT are the best-understood pathways. The STT segregates into medial and lateral branches near the thalamus with the medial branch terminating in medial thalamus and the lateral branch terminating in lateral thalamus. The lateral branch is known also as the neospinothalamic pathway. The medial branch is known also as the paleospinothalamic pathway or the paramedian pathway. The paleospinothalamic pathway also sends a collateral pathway to the reticular formation and appears functionally similar to the SRT.6 Each of four distinct thalamic nuclei, which receive nociceptive input from the spinal cord, has projections to the cerebral cortex, anterior cingulate cortex or to the insula. The primary somatosensory cortex has neurons responding selectively to nociceptive input. Recent studies with PET (positron emission tomography) imaging show that the somatosensory cortex is important for interpretation of pain location, pattern, and possibly intensity.23� The frontal cortex receives projections from the central lateral and submedial thalamic nuclei.1 PET studies show the frontal cortex and especially the anterior cingulate cortex to be involved in affective components of pain. Additionally, research shows the insula is involved in the suffering components of pain.24 Recent evidence refutes the common belief that a single brain structure is responsible for pain perception. Transmission of the action potential in the second-order neuron by way of the anterolateral spinal cord quadrant can be interrupted by anterolateral cordotomy. This procedure can be performed by open surgical or percutaneous techniques, both of which create a spinal cord lesion that transects axons ascending to the brain in the anterolateral quadrant.25 Theoretically the procedure is appealing, but clinical results have been disappointing for many people. Careful pre-procedure selection improves results. Good pain relief is likely in people with cancer pain characterized by unilateral location below the mandible and with lancinating or toothache qualities rather than burning, pricking, pressure, or crawling qualities.26 Lack of complete pain relief with cordotomy may be related to sparing of axons in the anterolateral quadrant which overlap into the descending corticospinal tract or creation of a new neuropathic pain. The corticospinal neurons are involved with descending motor input and are crucial to functions such as bowel and bladder control, arm movement, and ambulation. If preservation of these motor functions is important, complete transection of the anterolateral quadrant fibers, which transmit nociceptive information rostrally, generally is not possible. Pain Perception Because of the complex neural mechanisms of nociceptive processing, pain is perceived as a multidimensional sensory and emotional experience to which there are cognitive and behavioral responses. Hence the acronym, the ABCs of Pain, serves as a means by which the distinctive components can be remembered easily by patients with pain and by health professionals. In particular, the sensory component of pain is paramount in appropriate assessment of pain perception. At minimum, sensory pain elements include pattern, area, intensity, and nature, which spell the word PAIN. Persons with pain, when provided with tools, easily report these four sensory pain elements. Sensory pain reports can be indispensable to appropriate diagnosis and treatment when a nurse knowledgeable about the pain process interprets the data. Persons with pain, however, are the most appropriate experts about the effectiveness of therapies prescribed to modulate the pain process and block pain perception. Modulation Fields and Basbaum30 proposed a diagram of the structural components of the descending opioid-related pain inhibitory system (Figure 11). Generally, findings indicate that several centers are involved in generating analgesia, three of which have received extensive investigation, the periventricular and periaqueductal grey, the rostral ventral medulla, and the spinal cord.� Afferent input to the descending pain modulating system is less well known, but certainly hypothalamic and amygdala inputs are involved and possibly the frontal granular and insular cortex.1�
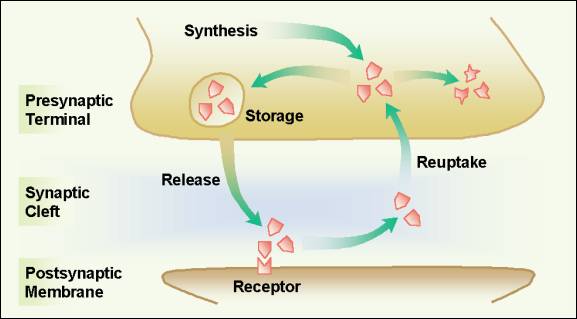
Figure 11.Descening pain modulation neurons form a network that extends from the fontal cortex and hypothalamus down through the periaqueductal grey (PGA) to the rostral ventral medulla and then down to the spinal dorsal horn.� rm=nucleus rahe magnus; mc=raticularis magnocellularis; pgl=paragigantocellularis; ne=noradrenergic pontomedullary cell groups; DLF=dorsolateral funiculus. � 2001 D.J. Wilkie, used with permission.� Descending inhibition of pain occurs through a complex circuit involving a number of receptor systems, such as mu, delta, and kappa opioid; alpha2 adrenergic; serotonin (5HT); adenosine; GABA; neuropeptide Y; calcitonin; somatostatin; and neurotensin receptors.1, 22 Although 5HT, alpha2 agonists, and opioids are known to inhibit nociceptive cells in the spinal dorsal horn, the role of the neurochemicals has not been fully delineated in each of the areas of the CNS that are believed to be involved in pain modulation. Figure 12 provides a graphical summary of the descending inhibitory mechanisms at the level of the spinal cord dorsal horn. Once nociceptive information is perceived as pain, inhibition can occur at any of the synapses in the ascending pathways. A well-studied and important inhibitory synapse is in the spinal dorsal horn. For example, serotonin, norepinephrine, and enkephalin are released by descending fibers and inhibit release of neurotransmitters, such as substance P and CGRP, and thereby diminish excitation of projection cells. The inhibitory neurotransmitters successfully prevent the PAN from communicating its information about the nociceptive stimuli to the second-order neuron and pain is blocked even though the PAN has been activated and has transmitted an action potential to the spinal cord. If the PAN action potential does not result in release of sufficient neurotransmitters to communicate the signal to the projection cell, pain is blocked. Figure 12. �Dorsal horn schematic illustration of local circuitry. High-threshold afferents (A-delta and C fibers and inhibitory interneurons (B) in lamina II0.� The excitatory interneuron provides further excitatotry drive to limina I projection cell whereas the inhibitory interneuron (B) provides a circuit that generates an inhibitory, feed-forward control of the lamina I projection cell by nociceptive inputs. Low-threshold primary afferent fibers provide a non-nociceptive input to lamina I projection cells via their excitatory connections with dendrites of excitatory cells (A) in lamina IIi.� In contract, the non-nociceptive input to inhibitory interneurons in lamina IIi(C) may contribute to the inhibitory control of nociceptive lamina I projection cells.� the schema also illustrates some possible descending control mechanisms. These may be exerted directly on dorsal horn lamina I-projection neurons. Alternatively, descending axons (some of which contain serotonin) may excite inhibitory interneuronw (e.g., enkephalin-containing (D) cells, which in turn postsynaptically control the nociceptive lamina I-projection neurons. Another possibility not illustrated is that the descending systems inhibit the excitatory cell (A).� Adapted from 20, p. 248�� 2001 D.J. Wilkie, used with permission.� Many drug and behavioral therapies provide pain relief though actions involving the descending pain inhibition mechanisms. Opioids, tricyclic antidepressants, alpha2 agonists, placebos, counter irritation, hypnosis, imagery, and distraction act via mechanisms to mimic or enhance descending inhibitory systems (Table 1). When these therapies are combined with methods that influence the process of PAN transduction and transmission, impressive analgesia can be obtained (Figure 13).� Opioids mimic the descending inhibitory system by binding to endogenous endorphin receptors (mu, delta, kappa) in the brain, spinal cord and peripheral tissues. Opioids act by hyperpolarizing the cell membrane and thereby inhibiting generation of an action potential.1, 22 Opioids effectively inhibit A-delta and C fibers, but are less effective in the wind-up state described previously.� One exception is methadone, which is believed to have antagonist activity at the NMDA receptor. Click to View Table 1.� Neural Mechanisms of Pain: Facilitating and Inhibiting Factors Figure 13. Putting the pain process together: A schematic showing sites of action of commonly used pharmacologic and behavioral analgesic therapies.�� Administered in small volumes by the intrathecal route, opioids exert powerful analgesic action at spinal cord synapses with limited rostral spread. In contrast, opioids delivered by the epidural route exert action not only at spinal cord sites, but also brain and peripheral sites because a substantial portion of the dose is absorbed by epidural blood vessels and provides access to systemic circulation. Opioids administered systemically (oral, transmucosal, transdermal, rectal, vaginal, subcutaneous, intramuscular, or intravenous) cross the blood brain barrier, enter the cerebrospinal fluid, and bind to opioid receptors throughout the brain and spinal cord. Discovery of opioid receptors on the PAN suggests that systemically administered opioids may exert analgesic action at peripheral sites in inflamed tissue as well as at central nervous system sites.31� Interdermal injection of opioids at the tissue injury site has shown impressive analgesia.1 Analgesic effects, side effects, and doses required to produce analgesia, will all differ based on route of administration, in part because access to opioid-receptors varies by administration route. Figure 14.� Inhibiting re-uptake of serotonin and norepinephrine enhances analesia of the tricyclic antidepressant drugs by keeping these chemicals in the synaptic cleft and available for binding to postsynaptic recptors that inhibit pain transmission. � 2001 D.J. Wilkie, used with permission. In comparison to opioids, tricyclic antidepressant drugs enhance the descending pain inhibitory system by preventing cellular re-uptake of serotonin and norepinephrine (Figure 14). These transmitters typically are released from the cell and rapidly transported back into the cell.� Rapid re-uptake limits the time that serotonin and norepinephrine are available for receptor binding and thereby to inhibit transmission of nociceptive signals. Tricyclic antidepressants, which have moderate serotonin effects and weak to potent norepinephrine effects32, 33 are effective adjuvant analgesics in some neuropathic pain conditions. Alpha2 agonists (e.g., clonidine), calcitonin, somatostatin, and baclofen are other agents known to provide analgesia.� Exact location of action for these agents is known for some but not others.� Clonidine, for example, acts pre- and post-synaptically, at alpha2 receptors in the dorsal horn, to hyperpolarize cell membranes and thereby to inhibit generation of the action potential.22 Baclofen also acts pre- and post-synaptically, but does so at GABA B receptors.� It is less clear where calcitonin and somatostatin act. Gabapentin interacts with an alpha2 delta subunit of a voltage-dependent Ca2+ channel.34 These agents also are known as adjuvant analgesics and are especially effective in neuropathic pain states. The exact mechanisms by which the behavioral therapies exert analgesia are not known.� It clearly has been demonstrated, however, that placebo response is mediated by endogenous opioid systems.Placebo response can be reversed by naloxone (Narcan), indicating that its mechanism involves enkephalin systems.35 It is possible that other behavioral therapies, such as counter-irritation or DNIC36 (descending noxious inhibitory control), hypnosis, imagery, and distraction also act via endogenous opioid or possibly non-opioid inhibitory systems throughout the neural axis (e.g., brain and spinal cord). Many of the inhibitory mechanisms that block pain transmission were initially proposed in general by Melzack and Wall37 in their gate control theory of pain. Today some of the details by which inhibition occurs are known and progress is being made to understand those not known. The message important for Nursing practice is that multiple methods can be used simultaneously to prevent transduction or transmission of nociceptive stimuli and therefore to block the perception of pain (Table 1). Differentiating nociceptive from neuropathic pain processes is important to therapy decisions. Used together several pharmacologic and behavioral therapies, each of which block pain perception via different mechanisms can dramatically improve pain relief. Genetic Alterations Table 2.� Genetic Alteration in Analgesic Drug Metabolism: Polymorphisms of Cytochrome P450 in which Enzyme Activity is Slightly, Moderately, or Completely Reduced.38-40 The CYP2D6 gene encodes an enzyme that breaks down 25% of all known drugs. The pain process includes neural mechanisms related to transduction, transmission, perception, and modulation. These mechanisms represent complex, not fully understood systems, but begin to explain the tremendous variability in pain reported by persons experiencing similar degrees of tissue damage. As previously described nearly 35 years ago by the Gate Control Theory of pain,37 the outcome of activation of nociceptive receptors is not totally predictable. The amount of pain perceived by an individual may vary tremendously depending on the context of the situation, including the person�s genetic capability to metabolize analgesic drugs. This context may include other physiological, sensory, affective, cognitive, or behavioral variables, the effects of which cannot be measured physiologically given the limitations of currently available methods. The person's perception of the pain, however, can be measured using a variety of pain scales. Information derived from the scales about the pattern, area, intensity, and nature of the pain as well as the emotional, behavioral, and cognitive responses to the pain provide the diagnostic foundation that the nurse. This information helps the nurse to recognize nociceptive and neuropathic types of pain and then to make clinical decisions about administering or suggesting appropriate pain relief therapies. Understanding about� the pain process and genetic alterations in drug metabolism helps the nurse to interpret pain assessment data and to implement multimodality interventions that maximize pain relief while minimizing side effects of therapies. Knowledge about neural mechanisms of nociception, pain perception, and pain modulation is vital for clinical decision making and rational management of pain experienced by seriously ill patients.
|